Background
Pulmonary embolism (PE) is a common and potentially lethal condition that can cause death in all age groups. A good clinician should consider the diagnosis if any suspicion of pulmonary embolism exists, because prompt diagnosis and treatment can dramatically reduce the morbidity and mortality of the disease. Unfortunately, the diagnosis is often missed, because pulmonary embolism frequently causes only vague and nonspecific symptoms.
The most sobering lessons about pulmonary embolism (PE) are those obtained from a careful study of the autopsy literature. Deep vein thrombosis (DVT) and pulmonary embolism are much more common than usually realized. In a long-range population cohort study, an equal number of venous thrombotic events were discovered after death, at autopsy, as were predicted by death certificate.1
The variability of presentation sets the patient and clinician up for potentially missing the diagnosis. The challenge is that the "classic" presentation with abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia is rarely the case. Studies of patients who die unexpectedly of pulmonary embolism reveal that they complained of nagging symptoms often for weeks before death related to pulmonary embolism. Forty percent of these patients had been seen by a physician in the weeks prior to their death.2
Pathophysiology
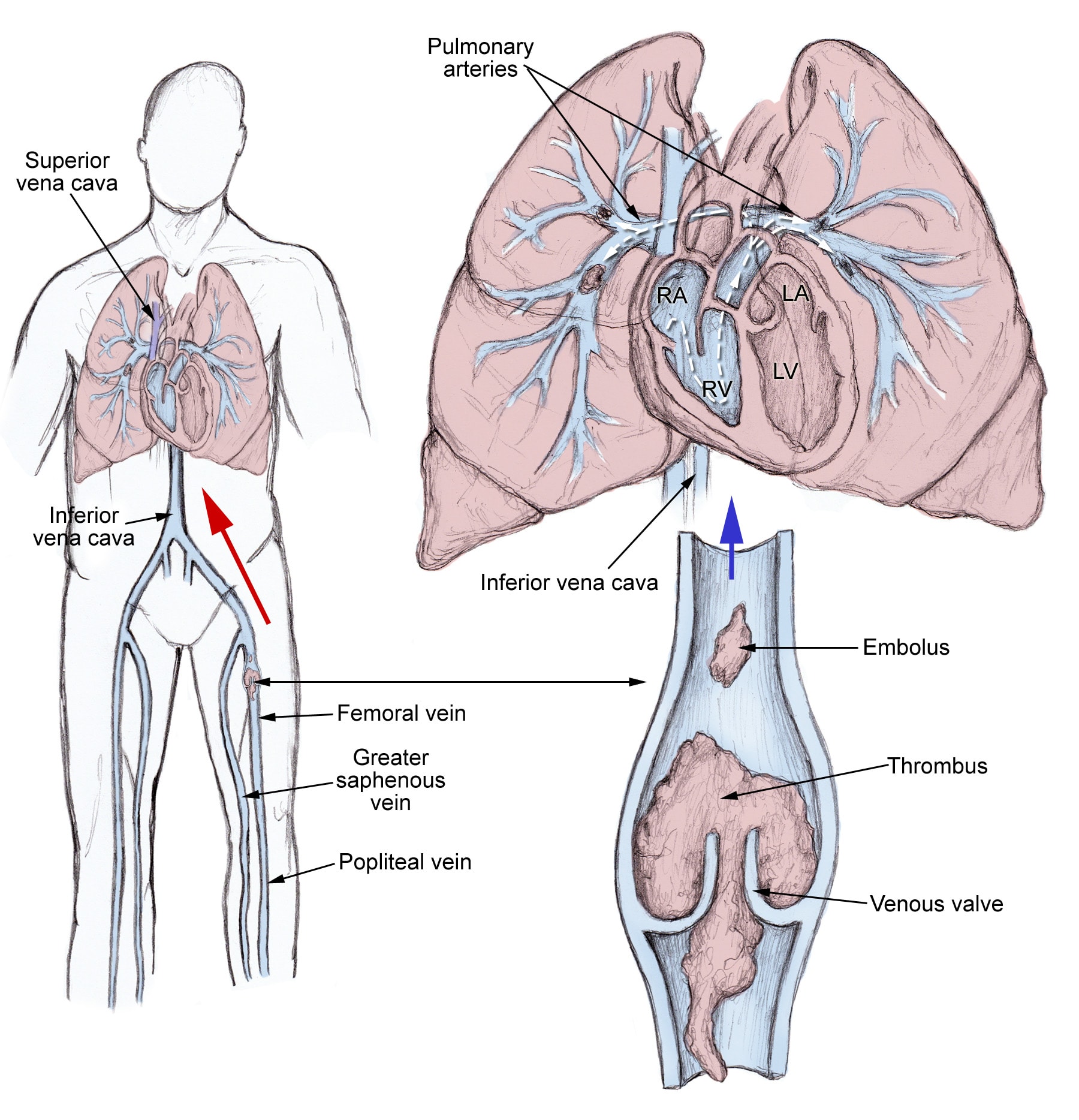
The pathophysiology of pulmonary embolism. Although pulmonary embolism can arise from anywhere in the body, most commonly it arises from the calf veins. The venous thrombi predominately originate in venous valve pockets (inset) and at other sites of presumed venous stasis. To reach the lungs, thromboemboli travel through the right side of the heart. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle.
Pulmonary thromboembolism is not a disease in and of itself. Rather, it is a complication of underlying venous thrombosis. Under normal conditions, microthrombi (tiny aggregates of red cells, platelets, and fibrin) are formed and lysed continually within the venous circulatory system. This dynamic equilibrium ensures local hemostasis in response to injury without permitting uncontrolled propagation of clot. Under pathological conditions, microthrombi may escape the normal fibrinolytic system to grow and propagate. Pulmonary embolism (PE) occurs when these propagating clots break loose and embolize to block pulmonary blood vessels.
Thrombosis in the veins is triggered by venostasis, hypercoagulability, and vessel wall inflammation. These 3 underlying causes are known as the Virchow triad. All known clinical risk factors for DVT and PE have their basis in one or more elements of the triad.
Patients who have undergone gynecologic surgery, those with major trauma, and those with indwelling venous catheters may have DVTs that start in an area related to their pathology. For other patients, venous thrombosis most often involves the lower extremities and nearly always starts in the calf veins, which are involved in virtually all cases of symptomatic spontaneous lower extremity DVT. Although DVT starts in the calf veins, in cases of pulmonary embolism, it will usually propagate proximally to the popliteal vessels, and from that area embolize.
Frequency
United States
Venous thromboembolism is a major health problem. The average annual incidence of venous thromboembolism in the United States is 1 per 1000,1,3,4 with about 250,000 incident cases occurring annually. The challenge in understanding the real disease is that autopsy studies show that an additional equal number of patients are diagnosed with pulmonary embolism at autopsy, as were initially diagnosed by clinicians.1,5 This is led to estimates of between 650,000 to 900,000 fatal and nonfatal VTE events occurring in the US annually. The incidence of venous thromboembolism has not changed significantly over the last 25 years.1 Capturing the true incidence going forward will be challenging because of the decreasing rate of autopsy. In a longitudinal, 25-year prospective study from 1966 to 1990, autopsy rates dropped from 55% to 30% over the study period.1 Current trends would suggest a continued decline in autopsy rate.
International
International journal articles cite similar population incidence of deep vein thrombosis and pulmonary embolism as the United States studies.
Mortality/Morbidity
Mortality for acute pulmonary embolism can be broken down into 2 categories: massive pulmonary embolism and nonmassive pulmonary embolism.
Massive pulmonary embolism is defined as presenting with a systolic arterial pressure less than 90 mm Hg. In two large international studies, this accounted for 4-4.5% of the patients. Nonmassive pulmonary embolism is defined as having a systolic arterial pressure greater than or equal to 90 mm Hg. This is the more common presentation for pulmonary embolism and accounts for 95.5-96% of the patients.6,7
The mortality for patients presenting with massive pulmonary embolism is between 30% and 60% depending on the study cited.8,7,3 The majority of these deaths occur in the first 1-2 hours of care, so it is important for the initial treating physician to have a systemized aggressive evaluation and treatment plan for patients presenting with pulmonary embolism. The diagnosis of massive pulmonary embolism is not solely a function of the size of the clot, rather it is a function of the size of the clot and the functional capability of the patient's cardiovascular system.
Hemodynamically stabile pulmonary embolism has a much lower mortality rate, especially in recent years, because of treatment with anticoagulant therapy. In nonmassive pulmonary embolism, the death rate is less than 5% in the first 3-6 months of anticoagulant treatment. The rate of recurrent thromboembolism is less than 5% during this time. However, recurrent thromboembolism reaches 30% after 10 years.9
Race
Studies looking at the incidence of pulmonary embolism in various races show that African American patients are the highest risk group, with a 50% higher incidence than American whites. Asian/Pacific Islanders/American Indian patients have a markedly lower risk of thromboembolism.9,10
Sex
Across all age groups, there is a fairly equal distribution of initial pulmonary embolism between males and females.1 However, most studies find that women have a significantly lower rate of recurrent pulmonary embolism.11
Age
Venous thromboembolism and pulmonary embolism are diseases associated with advancing age. Furthermore, pulmonary embolism accounts for a larger proportion of venous thromboembolic disease with increasing age for both sexes. This may well be the result of a cumulative effect of risk factors that patients acquire with aging.1,11
Clinical
History
Pulmonary embolism (PE) is so common and so lethal that the diagnosis should be sought actively in every patient who presents with any chest symptoms that cannot be proven to have another cause.
- Symptoms that should provoke a suspicion of pulmonary embolism must include chest pain, chest wall tenderness, back pain, shoulder pain, upper abdominal pain, syncope, hemoptysis, shortness of breath, painful respiration, new onset of wheezing, any new cardiac arrhythmia, or any other unexplained symptom referable to the thorax.
- The classic triad of signs and symptoms of PE (hemoptysis, dyspnea, chest pain) are neither sensitive nor specific. They occur in fewer than 20% of patients in whom the diagnosis of PE is made, and most patients with those symptoms are found to have some etiology other than PE to account for them. Of patients who go on to die from massive PE, only 60% have dyspnea, 17% have chest pain, and 3% have hemoptysis. Nonetheless, the presence of any of these classic signs and symptoms is an indication for a complete diagnostic evaluation.
- Many patients with PE are initially completely asymptomatic, and most of those who do have symptoms have an atypical presentation.
- Patients with PE often present with primary or isolated complaints of seizure, syncope, abdominal pain, high fever, productive cough, new onset of reactive airway disease ("adult-onset asthma"), or hiccoughs. They may present with new-onset atrial fibrillation, disseminated intravascular coagulation, or any of a host of other signs and symptoms.
- Pleuritic or respirophasic chest pain is a particularly worrisome symptom. PE has been diagnosed in 21% of young, active patients who come to the ED complaining only of pleuritic chest pain. These patients usually lack any other classical signs, symptoms, or known risk factors for pulmonary thromboembolism. Such patients often are dismissed inappropriately with an inadequate workup and a nonspecific diagnosis, such as musculoskeletal chest pain or pleurisy.
Physical
- Massive pulmonary embolism (PE) causes hypotension due to acute cor pulmonale, but the physical examination findings early in submassive PE may be completely normal.
- After 24-72 hours, loss of pulmonary surfactant often causes atelectasis and alveolar infiltrates that are indistinguishable from pneumonia on clinical examination and by radiography.
- New wheezing may be appreciated. If pleural lung surfaces are affected, a pulmonary rub may be heard.
- In patients with recognized PE, the incidence of physical signs has been reported as follows:
- 96% have tachypnea (respiratory rate >16/min)
- 58% develop rales
- 53% have an accentuated second heart sound
- 44% have tachycardia (heart rate >100/min)
- 43% have fever (temperature >37.8°C)
- 36% have diaphoresis
- 34% have an S 3 or S 4 gallop
- 32% have clinical signs and symptoms suggesting thrombophlebitis
- 24% have lower extremity edema
- 23% have a cardiac murmur
- 19% have cyanosis
Causes
As stated in the Pathophysiology section, the etiology of venous thrombosis and subsequent thromboembolism results from a distortion in Virchow's triad by venostasis, hypercoagulability, or vessel wall inflammation. These risk factors for venous thrombosis and pulmonary embolism can be broken down into hereditary factors and acquired factors.
- Hereditary factors (most result in a hypercoagulable state)
- Antithrombin III deficiency
- Protein C deficiency
- Protein S deficiency
- Factor V Leiden (most common genetic risk factor for thrombophilia)
- Plasminogen abnormality
- Plasminogen activator abnormality
- Fibrinogen abnormality
- Resistance to activated protein C
- Acquired factors (The most important clinically identifiable risk factors for DVT and PE are a prior history of DVT or PE, recent surgery or pregnancy, prolonged immobilization, or underlying malignancy.)
- Reduced mobility
- Fractures
- Immobilization
- Burns
- Obesity
- Old age
- Malignancy
- Chemotherapy
- Acute medical illness
- AIDS (lupus anticoagulant)
- Behçet disease
- Congestive heart failure (CHF)
- Myocardial infarction
- Polycythemia
- Systemic lupus erythematosus
- Ulcerative colitis
- Trauma/major surgery
- Spinal cord injury
- Catheters (indwelling venous infusion catheters)
- Postoperative
- Pregnancy
- Postpartum period
- Oral contraceptives
- Estrogen replacements (high dose only)
- Drug abuse (intravenous [IV] drugs)
- Drug-induced lupus anticoagulant
- Hemolytic anemias
- Heparin-associated thrombocytopenia
- Homocysteinemia
- Homocystinuria
- Hyperlipidemias
- Phenothiazines
- Thrombocytosis
- Varicose veins
- Venography
- Venous pacemakers
- Venous stasis
- Warfarin (first few days of therapy)
Differential Diagnoses
Workup
Laboratory Studies
- The challenge of evaluating laboratory studies and pulmonary embolism (PE) is that no one study can provide the answer. The clinical scoring guidelines seek to quantify the aforementioned risk factors to help guide decision-making with pulmonary embolism.
- Clinical scoring algorithms are less objective and less powerful than some authors would claim. The objective components of the Wells (Canadian Pulmonary Embolism Score) criteria, for example, have been shown to have little effect on the stratification power of the criteria; virtually all of the classification power is associated with a physician's subjective prejudgment of the likelihood of PE. The Geneva criteria, which depend only on objective measures, lead to a stratification with a PE prevalence of 8% in the lowest-risk group (Geneva score of zero)—a prevalence too high to be neglected. When PE is suspected, diagnostic tests must be performed.
- Unfortunately, no known blood or serum test can move a patient with a high clinical likelihood of pulmonary thromboembolism into a low likelihood category or vice versa.
- The PO2 on arterial blood gases analysis (ABG) has a zero or even negative predictive value in a typical population of patients in whom PE is suspected clinically. This is contrary to what has been taught in many textbooks, and it seems counterintuitive, but it is demonstrably true. The reason is as follows:
- Other etiologies that masquerade as PE are more likely to lower the PO2 than is PE. In fact, because other diseases that may masquerade as PE (eg, chronic obstructive pulmonary disease [COPD], pneumonia, CHF) affect oxygen exchange more than PE, the blood oxygen level often has an inverse predictive value for PE.
- In most settings, fewer than half of all patients with symptoms suggestive of PE actually turn out to have PE as their diagnosis. In such a population, if any reasonable level of PaO2 is chosen as a dividing line, the incidence of PE will be higher in the group with a PaO2 above the dividing line than in the group whose PaO2 is below the divider. This is a specific example of a general truth that may be demonstrated mathematically for any test finding with a Gaussian distribution and a population incidence of less than 50%.
- Conversely, in a patient population with a very high incidence of PE and a lower incidence of other respiratory ailments (eg, postoperative orthopedic patients with sudden onset of shortness of breath), a low PO2 has a strongly positive predictive value for PE.
- The discussion above holds true not only for arterial PO2 but also for the alveolar-arterial oxygen gradient and for the oxygen saturation level as measured by pulse oximetry. In particular, pulse oximetry is extremely insensitive, is normal in the majority of patients with PE, and should not be used to direct a diagnostic workup.
- The white blood cell (WBC) count may be normal or elevated. A WBC count as high as 20,000 is not uncommon in patients with PE.
- Clotting study results are normal in most patients with pulmonary thromboembolism.
- Prolongation of the prothrombin time (PT), activated partial thromboplastin time (aPTT), or clotting time have no prognostic value in the diagnosis of PE. DVT and PE can and often do recur in patients who are fully anticoagulated.
- New PE in the hospital occurs in the following despite therapeutic anticoagulation:
- Patients who have nonfloating DVT without PE at presentation (3%)
- Patients who present with a floating thrombus but no PE (13%)
- Patients who already had PE at presentation but had no floating thrombus (11%)
- Patients presenting with PE who have a floating thrombus visible at venography (39%)
- D-dimer is a unique degradation product produced by plasmin-mediated proteolysis of cross-linked fibrin. D-dimer is measured by latex agglutination or by an enzyme-linked immunosorbent assay (ELISA) and a test result is considered positive if the level is greater than 500 ng/mL.
- Latex agglutination tests are notoriously unreliable, with a historical sensitivity of only 50-60% for DVT and PE.
- The ELISA test is more sensitive than the latex agglutination test, with a sensitivity of 96-98%. The challenge is that the test is nonspecific and results may be positive in patients with infection, cancer, trauma, or other inflammatory states.
- A D-dimer screen is best used in conjunction with a clinical assessment of the patient's probability of pulmonary embolism. This can be done systematically using a scoring criteria12,13,14 or in a more gestalt style, basing the probability on the patient's history of predisposing conditions.15
Imaging Studies
- The initial chest radiographic findings of a patient with pulmonary embolism (PE) are virtually always normal, although on rare occasions, they may show the Westermark sign (ie, a dilatation of the pulmonary vessels proximal to an embolism along with collapse of distal vessels, sometimes with a sharp cutoff).
- Over time, an initially normal chest radiograph often begins to show atelectasis, which may progress to cause a small pleural effusion and an elevated hemidiaphragm.
- After 24-72 hours, one third of patients with proven PE develop focal infiltrates that are indistinguishable from an infectious pneumonia.
- A rare late finding of pulmonary infarction is the Hampton hump, a triangular or rounded pleural-based infiltrate with the apex pointed toward the hilum, frequently located adjacent to the diaphragm.
- Because chest radiography is unreliable, conduct high-resolution multidetector computed tomographic angiography (MDCTA) in patients suspected of having PE.
- MDCTA has been shown to have sensitivity and specificity comparable to that of contrast pulmonary angiography, and, in recent years, has become accepted both as the preferred primary diagnostic modality and as the criterion standard for making or excluding the diagnosis of pulmonary embolism.
- In many patients, multidetector CT scans with intravenous contrast can resolve third-order pulmonary vessels without the need for invasive pulmonary artery catheters.
- MDCTA is more likely to miss lesions in a patient with pleuritic chest pain due to multiple small emboli that have lodged in distal vessels, but these lesions also may be difficult to detect using conventional angiography.
- If MDCTA is unavailable, conduct pulmonary angiography. Long the criterion standard for PE diagnosis, pulmonary angiography is nevertheless more invasive and harder to perform than MDCTA and, for these reasons, is rapidly being replaced. Pulmonary angiography remains a useful diagnostic modality when MDCTA cannot be performed.
- When performed carefully and completely, a positive pulmonary angiogram provides virtually 100% certainty that an obstruction to pulmonary arterial blood flow does exist. A negative pulmonary angiogram provides greater than 90% certainty in the exclusion of PE.
- A positive angiogram is an acceptable endpoint no matter how abbreviated the study. However, a complete negative study requires the visualization of the entire pulmonary tree bilaterally. This is accomplished via selective cannulation of each branch of the pulmonary artery and injection of contrast material into each branch, with multiple views of each area. Even then, emboli in vessels smaller than third order or lobular arteries are not seen.
- Small emboli cannot be seen angiographically, yet embolic obstruction of these smaller pulmonary vessels is very common when postmortem examination follows a negative angiogram. These small emboli can produce pleuritic chest pain and a small sterile effusion even though the patient has a normal V/Q scan and a normal pulmonary angiogram.
- In most patients, however, PE is a disease of multiple recurrences, with both large and small emboli already present by the time the diagnosis is first suspected. Under these circumstances, both the V/Q scan and the angiogram are likely to detect at least some of the emboli.
- Until recently, nuclear scintigraphic ventilation-perfusion (V/Q) scanning of the lung had been the single most important diagnostic modality for detecting pulmonary thromboembolism available to the clinician. Other alternatives were less sensitive, less specific, or significantly more invasive. Multidetector CT angiography is now a preferred primary diagnostic modality, but the V/Q scan remains an important part of the evaluation when multidetector CT angiography is not available or not appropriate for the patient.
- V/Q scanning is indicated whenever the diagnosis of PE is suspected and no alternative diagnosis can be proved.
- A repeat V/Q scan is indicated before stopping anticoagulation in a patient with irreversible risk factors for DVT and PE, because recurrent symptoms are common and a reference "posttreatment" V/Q scan can serve as a new baseline for comparison, often sparing the patient the need for a future angiogram.
- The Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) classification scheme allows interpretation of the results of the V/Q scan in a meaningful way, but this standard classification is not used in its entirety at every institution. At some institutions, V/Q scan findings are never reported as normal no matter what the actual pattern of perfusion. This is unfortunate because normal perfusion is the scan pattern with the highest predictive value. Some institutions continue to report nondiagnostic V/Q patterns using obsolete and clinically confusing terminology, such as indeterminate, intermediate, or low probability.
- Diagnostic V/Q patterns classified as high probability or as normal perfusion may be relied upon to guide the clinical management of patients when the prior clinical assessment is concordant with the scan result.
- No matter what language is used, a nondiagnostic V/Q pattern is not an acceptable endpoint in the workup for pulmonary thromboembolism. Pulmonary angiography or another definitive test must be performed when the diagnosis remains uncertain.
- Normal V/Q scan
- No perfusion defects are seen.
- At least 2% of patients with PE have this pattern, and 4% of patients with this pattern have PE. This means that approximately 1 of every 25 patients sent home after a normal V/Q scan actually has a PE that has been missed. This is unfortunate, but risk-benefit analysis supports the idea that unless the presentation is highly convincing and no alternate diagnosis is demonstrable, a normal perfusion scan pattern often may be considered negative for PE.
- High-probability scan
- This includes scans with any of the following findings:
- Two or more segmental or larger perfusion defects with normal chest radiographs and normal ventilation
- Two or more segmental or larger perfusion defects where chest radiographic abnormalities and ventilation defects are substantially smaller than the perfusion defects
- Two or more subsegmental and one segmental perfusion defect with normal chest radiograph and normal ventilation
- Four or more subsegmental perfusion defects with normal chest radiograph and normal ventilation
- Forty-one percent of patients with PE have this pattern, and 87% of patients with this pattern have PE.
- In most clinical settings, a high-probability scan pattern may be considered positive for PE.
- This includes scans with any of the following findings:
- Nondiagnostic scan (with a pattern type that was formerly graded as low probability)
- This includes scans with any of the following findings:
- Small perfusion defects, regardless of number, ventilation findings, or chest radiographic findings
- Perfusion defects substantially smaller than a chest radiographic abnormality in the same area
- Matching perfusion and ventilation defects in less than 75% of one lung zone or in less than 50% of one lung, with a normal or nearly normal chest radiograph
- A single segmental perfusion defect with a normal chest radiograph, regardless of ventilation match or mismatch
- Nonsegmental perfusion defects
- Sixteen percent of patients with PE have this pattern, and 14% of patients with this pattern have PE. This pattern often is called "low probability," but the term is a misnomer: in a typical population, 1 in 7 patients with this pattern turn out to have a PE.
- This scan pattern is an indication for pulmonary angiography or some other definitive test. All patients suspected of PE who have a nondiagnostic scan must have PE definitively ruled out or some definitive alternative diagnosis made.
- This includes scans with any of the following findings:
- Nondiagnostic scan (with a pattern type that was formerly graded as "intermediate probability")
- Any V/Q abnormality not otherwise classified: Approximately 40% of patients with PE fall into this category, and 30% of all patients with this pattern have PE.
- This scan pattern is always an indication for pulmonary angiography or another definitive test to rule out PE. Failure to pursue the diagnosis further in these patients leads to disastrous outcomes.
- Duplex ultrasonography
- The diagnosis of PE can be proven by demonstrating the presence of a DVT at any site. Sometimes, this may be accomplished noninvasively, by using duplex ultrasonography.
- To look for DVT using ultrasonography, the ultrasound transducer is placed against the skin and then is pressed inward firmly enough to compress the vein being examined. In an area of normal veins, the veins are easily compressed completely closed, while the muscular arteries are extremely resistant to compression.
- Where DVT is present, the veins do not collapse completely when pressure is applied using the ultrasound probe.
- A negative ultrasound scan does not rule out DVT, because many DVTs occur in areas that are inaccessible to ultrasonic examination. Before an ultrasound scan can be considered negative, the entire deep venous system must be interrogated using centimeter-by-centimeter compression testing of every vessel.
- In two thirds of patients with PE, the site of DVT cannot be visualized by ultrasound, so a negative duplex ultrasound scan does not markedly reduce the likelihood of PE.
Other Tests
- Electrocardiography
- The most common ECG abnormalities in the setting of pulmonary embolism (PE) are tachycardia and nonspecific ST-T wave abnormalities. The finding of S 1 Q 3 T 3 is nonspecific and insensitive in the absence of clinical suspicion for PE.
- The classic findings of right heart strain and acute cor pulmonale are tall, peaked P waves in lead II (P pulmonale), right axis deviation, right bundle-branch block, an S 1 Q 3 T 3 pattern, or atrial fibrillation. Unfortunately, only 20% of patients with proven PE have any of these classic ECG abnormalities.
- If ECG abnormalities are present, they may be suggestive of PE, but the absence of ECG abnormalities has no significant predictive value.
- Echocardiography or cardiac ultrasonography16
- The subcostal view is preferred at initial screening for mechanical activity and pericardial fluid and for gross assessment of global and regional abnormalities. To obtain a subcostal view, place the transducer the left subcostal margin with the beam aimed at the left shoulder.
- The parasternal view allows visualization of the aortic valve, proximal ascending aorta, and posterior pericardium and allows determination of left ventricular size. It is particularly helpful when the subcostal view is difficult to obtain. To obtain a parasternal view, place the transducer in the left parasternal area between the second and fourth intercostal spaces. The plane of the beam is parallel to a line drawn from the right shoulder to the left hip.
- Several echocardiographic findings have been proposed for noninvasive diagnosis of RV dysfunction at the bedside, including RV enlargement and/or hypokinesis of the free wall, leftward septal shift, and evidence of pulmonary hypertension. If right ventricular dysfunction is seen on cardiac ultrasonography, the diagnosis of acute submassive or massive PE is supported. While the presence of RV dysfunction can be used to support the clinical suspicion of PE, prognostic information can be obtained by assessing the severity of RV dysfunction.
- Under investigation
- Prompt diagnosis and stratification in patients with suspected PE and a high risk of adverse events may help to improve outcomes. Serum troponin, although seemingly marginal for purposes of diagnosis of PE, may contribute significantly to the ability to stratify patients by risk for short-term death or adverse outcome events when they reach the ED. In patients with PE and normal blood pressure specifically, elevated serum troponin level has been associated with right ventricular overload.17,18,19,20
- Elevated levels of brain-type natriuretic peptides (BNP) may also provide prognostic information.19 A recent meta-analysis demonstrated a significant association between elevated N-terminal–pro-BNP (NT-pro-BNP) and right ventricular function in patients with PE (p<0.001),>21 Importantly, increased NT-pro-BNP alone does not justify more invasive treatment.
- An potential alternative to D-dimer is ischemia-modified albumin (IMA) level, which data suggest, is 93% sensitive and 75% specific for PE.22 Notably, in a recent study comparing the prognostic value of IMA to D-dimer, IMA in combination with Wells and Geneva probability scores appears to positively impact overall sensitivity and negative predictive value.22 The positive predictive value of IMA, in particular, is better than D-dimer. However, it should not be used alone and, apparently, is still unable to confirm a PE diagnosis with further investigation.23
Procedures
- The primary indication for placement of an inferior vena cava filter in the setting of pulmonary embolism include contraindications to anticoagulation, major bleeding complications during anticoagulation, and recurrent embolization while the patient is receiving adequate therapy.
Treatment
Prehospital Care
- The most important thing that can be done in the prehospital setting is to transport the patient to a hospital. As long as no reliable method is available of making a clinical diagnosis of pulmonary embolism (PE) without diagnostic tests, treating PE in a meaningful way in the field will remain difficult.
- Isolated case reports exist of patients who have been resuscitated successfully after receiving fibrinolytic agents in the field for cardiac arrest strongly believed (and later proven) to be due to PE.
- Presumptive fibrinolysis in the field is aggressive, but it may be a reasonable course of action today when patients being treated as outpatients for known DVT suddenly become short of breath and hypotensive.
- Oxygen always should be started in the prehospital phase, and an IV line should be placed if it can be accomplished rapidly without delaying transport. Fluid loading should be avoided unless the patient's hemodynamic condition is deteriorating rapidly, because IV fluids may worsen the patient's condition. Without invasive testing or trial and surveillance, the physician cannot know whether additional preload will help or hurt a heart that is failing already because of high outflow pressures from pulmonary vascular obstruction.
Emergency Department Care
- Fibrinolytic therapy has been the standard of care for patients with massive or unstable pulmonary embolism (PE) since the 1970s. Unless overwhelming contraindications are evident, a rapidly acting fibrinolytic agent should be administered immediately to every patient who has suffered hypotension (even if resolved) or is significantly hypoxemic from PE.
- Improvement of hypotension in response to hydration or pressors does not remove the indication for immediate fibrinolysis. The fact that hypotension has occurred at all is a sufficient indication that the patient has exhausted his or her cardiopulmonary reserves and is at high risk for sudden collapse and death.
- Fibrinolysis also is indicated for patients with PE who have any evidence of right heart strain, because evidence indicates that the mortality rate can be cut in half by early fibrinolysis in this patient population.
- Today, fibrinolysis may be considered for any patient with PE who lack specific contraindications to the therapy. Some centers now regard fibrinolysis as the primary treatment of choice for all patients with PE. Interventional radiology has made it possible to perform transcatheter fibrinolysis for patients who have DVT without evidence of PE.
- Heparin reduces the mortality rate of PE because it slows or prevents clot progression and reduces the risk of further embolism.
- Heparin does nothing to dissolve clot that has developed already, but it is still the single most important treatment that can be provided, because the greatest contribution to the mortality rate is the ongoing embolization of new thrombi. Prompt effective anticoagulation has been shown to reduce the overall mortality rate from 30% to less than 10%.
- Early heparin anticoagulation is so essential that heparin should be started as soon as the diagnosis of significant pulmonary thromboembolism is considered.
- Oxygen should be administered to every patient with suspected PE, even when the arterial PO2 is perfectly normal, because increased alveolar oxygen may help to promote pulmonary vascular dilatation.
- IV fluids may help or may hurt the patient who is hypotensive from PE depending on which point on the Starling curve describes the patient's condition.
- A Swan-Ganz catheter is helpful to determine whether a fluid bolus is indicated; as an alternative, a cautious trial of a small fluid bolus may be attempted, with careful surveillance of the systolic and diastolic blood pressures and immediate cessation if the situation worsens after the fluid bolus.
- Improvement or normalization of blood pressure after fluid loading does not mean the patient has become hemodynamically stable.
- Fibrinolysis is indicated for any patient with a PE large enough to cause hypotension, even if the hypotension is transient or correctable. As noted above, early fibrinolysis may reduce the mortality rate by 50% for patients who have right ventricular dysfunction due to PE, even if they are hemodynamically stable.
- Compression stockings
- Compression stockings that provide a 30-40 mm Hg compression gradient should be used, because they are a safe and effective adjunctive treatment that can limit or prevent extension of thrombus.
- True gradient compression stockings (30-40 mm Hg or higher) are highly elastic, providing a gradient of compression that is highest at the toes and gradually decreases to the level of the thigh. This reduces capacitive venous volume by approximately 70% and increases the measured velocity of blood flow in the deep veins by a factor of 5 or more. Compression stockings of this type have been proven effective in the prophylaxis of thromboembolism and are also effective in preventing progression of thrombus in patients who already have DVT and PE.
- A 1994 meta-analysis calculated a DVT risk odds ratio of 0.28 for gradient compression stockings (as compared to no prophylaxis) in patients undergoing abdominal surgery, gynecologic surgery, or neurosurgery.
- Other studies have found that gradient compression stockings and low-molecular-weight heparin (LMWH) were the most effective modalities in reducing the incidence of DVT after hip surgery; they were more effective than subcutaneous unfractionated heparin, oral warfarin, dextran, or aspirin.
- The ubiquitous white stockings known as "anti-embolic stockings" or "Ted hose" produce a maximum compression of 18 mm Hg. Ted hose rarely are fitted in such a way as to provide even that inadequate gradient compression. Because they provide such limited compression, they have no efficacy in the treatment of DVT and PE, nor have they been proven effective as prophylaxis against a recurrence.
- True 30-40 mm Hg gradient compression pantyhose are available in sizes for pregnant women. They are recommended by many specialists for all pregnant women because they not only prevent DVT, but they also reduce or prevent the development of varicose veins during pregnancy.
Consultations
- Fibrinolytic therapy should not be delayed while consultation is sought. The decision to treat PE by fibrinolysis is properly made by the responsible emergency physician alone, and fibrinolytic therapy is properly administered in the ED.
- An interventional radiology consultation may be helpful for catheter-directed fibrinolysis in selected patients. In rare cases, arranging for placement of a venous filter may be appropriate if the patient is not a candidate for thrombolytic therapy.
Medication
Immediate full anticoagulation is mandatory for all patients with suspected deep vein thrombosis (DVT) or pulmonary embolism (PE) because effective anticoagulation with heparin reduces the mortality rate of PE from 30% to less than 10%. Heparin works by activating antithrombin III to slow or prevent the progression of DVT and to reduce the size and frequency of PE. Heparin does not dissolve existing clot.
Anticoagulation is essential, but anticoagulation alone does not guarantee a successful outcome. DVT and PE may recur or extend despite full and effective heparin anticoagulation.
Fibrinolytic therapy should be considered for 3 groups of patients: those who are hemodynamically unstable, those with right heart strain and exhausted cardiopulmonary reserves, and those who are expected to have multiple recurrences of pulmonary thromboembolism over a period of years. Patients with a prior history of PE and those with known deficiencies of protein C, protein S, or antithrombin III should be included in this latter group.
Fibrinolysis should be considered as a potential therapy for every patient with proven PE.
Long-term anticoagulation is essential for patients who survive an initial DVT or PE. The optimum total duration of anticoagulation has been controversial in recent years, but general consensus holds that at least 6 months of anticoagulation is associated with significant reduction in recurrences and a net positive benefit.
Fibrinolytics
Fibrinolysis is always indicated for hemodynamically unstable patients with PE, because no other medical therapy can improve acute cor pulmonale quickly enough to save the patient's life.
Because it is less invasive and has fewer complications, fibrinolytic therapy has replaced surgical embolectomy as the primary mode of treatment for hemodynamically unstable patients with pulmonary thromboembolism. Surgical thromboembolectomy now is reserved for patients in whom fibrinolysis has failed or cannot be tolerated.
Fibrinolytic regimens currently in common use for PE include 2 forms of recombinant tissue plasminogen activator, t-PA (alteplase) and r-PA (reteplase), along with urokinase and streptokinase. Alteplase usually is given as a front-loaded infusion over 90 or 120 minutes. Urokinase and streptokinase usually are given as infusions over 24 hours or more. Reteplase is a new-generation thrombolytic with a longer half-life that is given as a single bolus or as 2 boluses administered 30 minutes apart.
Of the 4, the faster-acting agents reteplase and alteplase are preferred for patients with PE, because the condition of patients with PE can deteriorate extremely rapidly.
Many comparative clinical studies have shown that administration of a 2-hour infusion of alteplase is more effective (and more rapidly effective) than urokinase or streptokinase over a 12-hour period. One prospective randomized study comparing reteplase and alteplase found that total pulmonary resistance (along with pulmonary artery pressure and cardiac index) improved significantly after just 0.5 hours in the reteplase group as compared to 2 hours in the alteplase group. Fibrinolytic agents do not seem to differ significantly with respect to safety or overall efficacy.
Streptokinase is least desirable of all the fibrinolytic agents because antigenic problems and other adverse reactions force the cessation of therapy in a large number of cases.
Empiric thrombolysis may be indicated in selected hemodynamically unstable patients, particularly when the clinical likelihood of PE is overwhelming and the patient's condition is deteriorating. The overall risk of severe complications from thrombolysis is low and the potential benefit in a deteriorating patient with PE is high. Empiric therapy especially is indicated when a patient is compromised so severely that he or she will not survive long enough to obtain a confirmatory study. Empiric thrombolysis should be reserved, however, for cases that truly meet these definitions, as many other clinical entities (including aortic dissection) may masquerade as PE, yet may not benefit from thrombolysis in any way.
If indicated, fibrinolysis may be used in pregnancy at the same dose used for nonpregnant patients. Fear of complications should not prevent the use of fibrinolytics when a pregnant patient has significant right ventricular dysfunction from PE, as the best predictor of fetal outcome in this setting remains maternal outcome.
Reteplase (r-PA, Retavase)
Second-generation recombinant tissue-type plasminogen activator. As fibrinolytic agent, seems to work faster than its forerunner, alteplase, and also may be more effective in patients with larger clot burden. Also has been reported more effective than other agents in lysis of older clots.
Two major differences help explain these improvements. Compared to alteplase, reteplase does not bind fibrin so tightly, allowing drug to diffuse more freely through clot. Another advantage seems to be that reteplase does not compete with plasminogen for fibrin-binding sites, allowing plasminogen at site of clot to be transformed into clot-dissolving plasmin.
FDA has not approved reteplase for use in PE.
Studies of reteplase for PE have used same dose approved by FDA for coronary artery fibrinolysis.Anticoagulants
Heparin augments the activity of antithrombin III and prevents the conversion of fibrinogen to fibrin. Full-dose LMWH or full-dose unfractionated IV heparin should be initiated at the first suspicion of DVT or PE.
With proper dosing, several LMWH products have been found safer and more effective than unfractionated heparin both for prophylaxis and for treatment of DVT and PE. Monitoring the aPTT is neither necessary nor useful when giving LMWH, because the drug is most active in a tissue phase and does not exert most of its effects on coagulation factor IIa.
Many different LMWH products are available around the world. Because of pharmacokinetic differences, dosing is highly product specific. Several LMWH products are approved for use in the United States: enoxaparin (Lovenox), dalteparin (Fragmin), and tinzaparin (Innohep). Enoxaparin and tinzaparin are currently approved by the FDA for treatment of DVT. Dalteparin is FDA approved for prophylaxis and has approval for cancer patients. Each of the other agents has been approved by the FDA at a lower dose for prophylaxis, but all appear to be safe and effective at some therapeutic dose in patients with active DVT or PE.
Fractionated LMWH administered subcutaneously is now the preferred choice for initial anticoagulation therapy. Unfractionated IV heparin can be nearly as effective but is more difficult to titrate for therapeutic effect. Warfarin maintenance therapy may be initiated after 1-3 days of effective heparinization.The weight-adjusted heparin dosing regimens that are appropriate for prophylaxis and treatment of coronary artery thrombosis are too low to be used unmodified in the treatment of active DVT and PE. Coronary artery thrombosis does not result from hypercoagulability but rather from platelet adhesion to ruptured plaque. In contrast, patients with DVT and PE are in the midst of a hypercoagulable crisis, and aggressive countermeasures are essential to reduce mortality and morbidity rates.
Warfarin (Coumadin)
Interferes with hepatic synthesis of vitamin K-dependent coagulation factors. Never give to patient with thrombosis until after patient has been anticoagulated fully with heparin, because first few days of warfarin therapy produce hypercoagulable state. Failing to anticoagulate with heparin before starting warfarin will cause clot extension and recurrent thromboembolism in about 40% of patients, compared with 8% of those who receive full-dose heparin before starting warfarin. Heparin should be continued for first 5-7 d of oral warfarin therapy, regardless of PT, to allow time for depletion of procoagulant vitamin K–dependent proteins.
Anticoagulant effect of warfarin adjusted by varying dose to keep INR within target range. An INR target range of 2.5 to 3.5 makes sense for DVT and PE because rate of recurrence increases dramatically when INR drops below 2.5 and decreases when INR is kept above 3.0. The risk of serious bleeding (including hemorrhagic stroke) is approximately constant when INR is between 2.5 and 4.5 but rises dramatically when INR is 5.0 or higher. In UK, higher INR target of 3.0 - 4.0 is recommended more often. Best evidence suggests that 6 mo of anticoagulation reduces rate of recurrence to half of that observed when only 6 wk of anticoagulation given.
Long-term anticoagulation indicated for patients with irreversible underlying risk factor with recurrent DVT or recurrent PE.
Procoagulant vitamin K–dependent proteins responsible for transient hypercoagulable state when warfarin first started and when stopped. This phenomenon occasionally causes warfarin-induced necrosis of large areas of skin or of distal appendages. Heparin always used to protect against this hypercoagulability when warfarin started, but when warfarin stopped, problem resurfaces, causing abrupt temporary rise in rate of recurrent venous thromboembolism.
At least 186 different foods and drugs have been reported to interact with warfarin. Clinically significant interactions have been verified for a total of 26 common drugs and foods, including 6 antibiotics and 5 cardiac drugs. Every effort should be made to keep patient adequately anticoagulated at all times because procoagulant factors recover first when warfarin therapy is inadequate.
Patients who have difficulty maintaining adequate anticoagulation while taking warfarin may be asked to limit their intake of foods that contain vitamin K. Foods that have moderate to high amounts of vitamin K include brussel sprouts, kale, green tea, asparagus, avocado, broccoli, cabbage, cauliflower, collard greens, liver, soybean oil, soybeans, certain beans, mustard greens, peas (black-eyed peas, split peas, chick peas), turnip greens, parsley, green onions, spinach, and lettuce.Follow-up
Further Inpatient Care
- Any degree of hemodynamic compromise or hypoxemia in a patient with pulmonary embolism (PE) is an indication that the patient should be assigned to a monitored unit rather than to a regular floor bed. These patients have exhausted their cardiopulmonary reserves and, because PE is a condition of many frequent recurrences, many of these patients may worsen suddenly at some point during their hospitalization.
Further Outpatient Care
- Outpatient treatment after diagnosis of pulmonary embolism consists of anticoagulation for 3 months. This is typically done by 5 days of either happening or low molecular weight heparin started in the hospital, followed by warfarin treatment for an INR of 2. In a study comparing the advantages of 3 to 6 months treatment with anticoagulation, no additional benefit was found; however, there were more bleeding-related complications.24
Deterrence/Prevention
- Preventing idiopathic outpatient pulmonary embolism is difficult if not impossible. That said, the majority of pulmonary embolism occurs in hospitalized patients, and their incidence of pulmonary embolism can be reduced by providing the patient with appropriate prophylaxis. This can be done with heparin, low molecular weight heparin, warfarin, or mechanical prophylaxis.25
Patient Education
- For excellent patient education resources, visit eMedicine's Lung and Airway Center and Circulatory Problems Center. Also, see eMedicine's patient education articles Pulmonary Embolism and Blood Clot in the Legs.
Miscellaneous
Medicolegal Pitfalls
- Because pulmonary embolism (PE) is both extremely common and fairly difficult to diagnose, many patients are seen in the ED and later die from undiagnosed PE. In fact, respiratory complaints are the most common complaints in patients who are seen alive in the ED and later die unexpectedly.
- A small number of often repeated mistakes in diagnosis and treatment are responsible for a large proportion of the bad outcomes with serious legal repercussions. The most common and most serious of these errors are as follows:
- Dismissing complaints of unexplained shortness of breath as anxiety or hyperventilation without an adequate workup
- Dismissing complaints of unexplained chest pain as musculoskeletal pain without an adequate workup
- Failure to properly diagnose and treat symptomatic deep vein thrombosis (DVT)
- Failure to recognize that DVT below the knee is just as serious as more proximal DVT
- Failure to order a CTPA or V/Q scan when a patient has symptoms consistent with PE
- Failure to start full-dose heparin at the first real suspicion of PE, before the V/Q scan
- Failure to give fibrinolytic therapy immediately when a patient with PE becomes hemodynamically unstable
Special Concerns
- Pregnancy
- Deep vein thrombosis (DVT) and pulmonary embolism (PE) are common during all trimesters of pregnancy and for 6-12 weeks after delivery.
- The diagnostic approach should be exactly the same in a pregnant patient as in a nonpregnant one. A nuclear perfusion lung scan is safe in pregnancy. A chest CT is safe in pregnancy. Heparin is safe in pregnancy. Fibrinolysis is safe in pregnancy. Failure to treat the mother properly is the most common cause of fetal demise.
- Geriatric
- PE becomes increasingly common with age, yet the diagnosis of PE is missed more often in the geriatric population, largely because respiratory symptoms often are dismissed as chronic in geriatric patients.
- Even when the diagnosis is made, appropriate therapy more often is withheld inappropriately in this population than in any other group.
- Reduced mobility
No comments:
Post a Comment